As humanity continues to release more and more carbon dioxide into our atmosphere, much is absorbed by the marine environment causing the sea to become more acidic over time through a process called ocean acidification. Ocean acidification (OA) happens when carbon dioxide dissolves in water creating carbonic acid which then dissociates to bicarbonate and a free hydrogen atom. The addition of all these free hydrogen atoms is what is slowing causing our oceans to become more acidic. However, some of these free hydrogen atoms are also reacting with carbonate ions to make bicarbonate. This process is shown below:
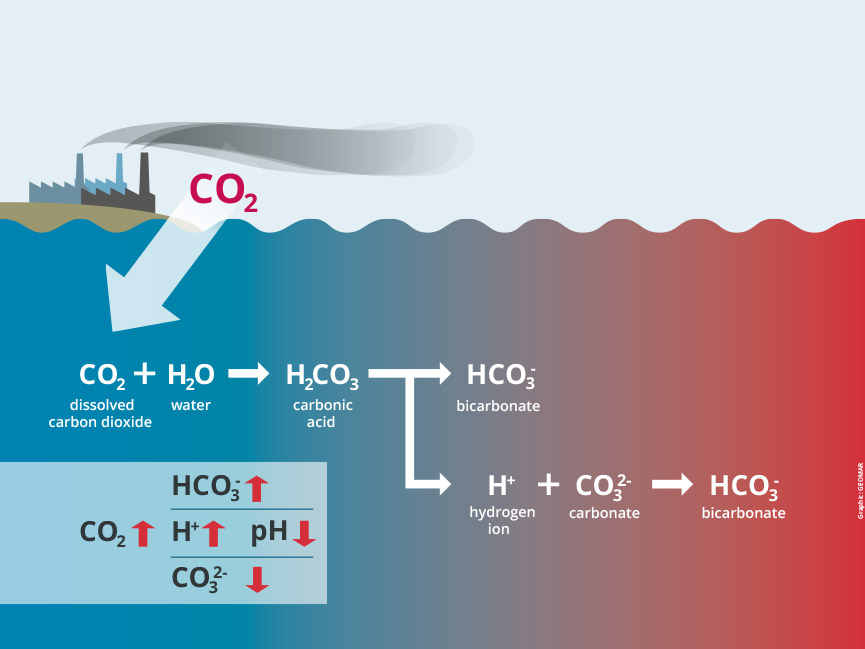
The figure above shows the process of ocean acidification through absorption of CO₂ into the water column. It starts with carbon dioxide being mixed with water to create carbonic acid which then dissociates into a free hydrogen atom and bicarbonate. These free hydrogen atoms then find carbonate to bond with creating more bicarbonate. The free floating hydrogen atoms are what increase the ocean acidity and when they react with carbonate to make even more bicarbonate it results in less carbonate in the water column for organisms to use for things such as creating a shell or skeleton. Creative Commons License, John P. Rafftery [1].
H₂CO₃ ↔ H⁺ + HCO₃⁻ (bicarbonate)
H⁺ + CO₃²⁻ (carbonate) ↔ HCO₃⁻
As carbonate becomes less abundant in the ocean, many organisms struggle to get a hold of it causing problems such as weaker shells, skeletons, and other protective structures. Think of it like vitamin C: All humans need it, but when we don’t get as much as we need we have to take supplements. Organisms that rely on carbonate are called calcifying organisms and they are very important when it comes to the formation of limestone. This is because limestone is sedimentary rock made up of calcium carbonate from shells or skeletal matter from animals such as coral, snails, and crabs. Even some types of plankton are made up of calcium carbonate and play a large role in forming limestone such as coccolithophores! All of this calcium carbonate under the immense pressure of our deep ocean is slowly pushed together over time creating a solid surface or rock called limestone.

The image above shows the rock limestone. Limestone is a common rock that is found in marine environments and is mostly made up of crystalline calcium carbonate. Over time this crystalline calcium carbonate can react with acidic pH levels to become amorphous calcium carbonate (meaning it has no clearly defined chemical structure). Creative Common License, James St. John [2].
So, how does OA affect the formation of limestone? With less carbonate available, organisms must work harder to create calcium carbonate which reduces calcification and precipitation rates. As these rates are reduced, the loss of calcium carbonate precipitation causes an increased reliance on things such as seed crystals, mineral fragments, or biological structures like proteins to lead crystal growth in order to overcome energy barriers. This can result in limestone with lower density and a more porous microstructure as well as more amorphous phases due to organisms producing more amorphous calcium carbonate. Organisms begin producing more amorphous calcium carbonate because this requires less energy and in an environment where carbonate is becoming increasingly difficult to find, it’s important they conserve as much energy as possible.
In conclusion, as our oceans take up more and more carbon from our atmosphere, less carbonate becomes available to calcifying organisms. Since limestone is largely made up of precipitation of calcium carbonate from biotic factors, the precipitation rates drop. This causes the formation of limestone with lower calcium and carbonate content and more amorphous and unstable phases. It also affects trace element ratios such as magnesium:calcium and strontium:calcium due to the different precipitation conditions. With less efficient calcification the limestone may also contain more organic matter or clays and overall be more porous and therefore less dense.